As global fire safety standards evolve and the incidence of industrial, residential, and vehicular fires remains a critical concern, the market for advanced fire suppression aerosols is expanding rapidly. These compact, portable systems deliver potent extinguishing agents in a fine mist, enabling quick response, minimal residue, and effective control over various fire classes without significant environmental harm.

Manufacturers aiming to capitalize on this demand must prioritize precision engineering, stringent quality controls, and adherence to international standards like NFPA and UL to produce reliable fire suppression aerosols that perform under pressure—literally and figuratively.
At Aile Aerosol, we specialize in bespoke filling solutions engineered for fire suppression aerosol production, blending cutting-edge technology with safety-focused design to ensure every unit is ready for real-world emergencies.
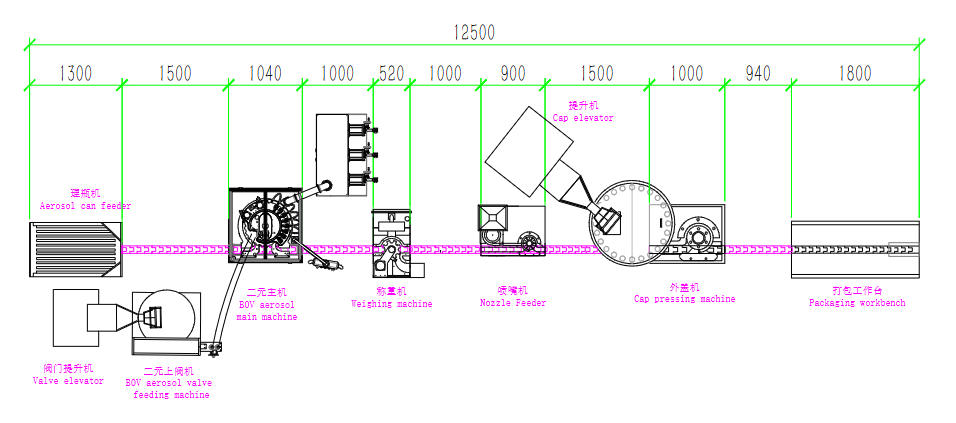
Understanding the Asthma Aerosol Manufacturing Process:
Asthma inhalers are complex systems consisting of:
Active ingredients such as Salbutamol sulfate or Terbutaline sulfate
Propellants (e.g., HFA-134a or HFA-227ea)
Additives including co-solvents, surfactants, and stabilizers
Each stage of production requires rigorous control to ensure product stability and consistent dose delivery.
Key Steps in MDI Production:
Container & Valve Preparation
All aluminum cans and metering valves are sterilized and dried to eliminate any risk of contamination before filling.
Formulation & Liquid Filling
Depending on the formulation type (solution, suspension, or emulsion), the drug is precisely blended and filled in controlled conditions to achieve uniformity and clarity.
Propellant Filling – Pressure or Cold Method
Pressure Filling: Propellant is injected into sealed cans at ambient temperature under controlled pressure — ideal for large-scale, efficient production.
Cold Filling: The propellant is cooled to sub-zero temperatures before filling — suitable for specific formulations but requires low-temperature systems.
Leak Testing & Final Packaging
Each filled can undergoes 100% leak and weight testing to ensure consistency, accuracy, and safety before labeling and packing.
Aile Aerosol Solutions for Pharmaceutical Aerosols:
Designed for high-precision pharmaceutical aerosol filling, the AILE-EYQWX integrates all critical functions into a single automated platform — from liquid dosing and valve placement to crimping and propellant filling.
It’s the ideal solution for continuous, high-output MDI manufacturing environments.
| Parameter | Specification |
|---|---|
| Sealing diameter(mm) | 26.5-27.5 |
| Sealing depth(mm) | 5.0-6.0 |
| Filling speed | 1200-1800 cans/h |
| Maximum liquid filling capacity | 300ml |
| Working pressure (MPa) | 0.5-0.7Mpa |
| Liquid repeat filling accuracy | ≤±1.0% |
The AILE-EYQWJ is purpose-built for new drug development, clinical trial production, and small-batch runs.
It combines liquid filling, sealing, and gas charging on one compact station — reducing manual intervention while maintaining high accuracy.
| Parameter | Specification |
|---|---|
| Material | SUS316 |
| Bottle Size | diamter35-70mm; height 80-330mm |
| Filling speed | 700-900 cans/h |
| Filling range | 30-500ml |
| Air pressure | 0.6-0.8Mpa |
| Filling Accuracy | within 1% |
Why Choose Aile Aerosol:
20+ years of experience in aerosol filling line integration
Proven expertise in pharmaceutical-grade small-dose applications
GMP and ISO certified design and manufacturing
Custom engineering support for Salbutamol, Terbutaline, and similar MDI products
End-to-end technical service — from machine setup to process validation
Whether you’re expanding your capacity or launching a new inhalation product, Aile Aerosol delivers precision-engineered equipment and turnkey solutions tailored to your pharmaceutical needs.
📩 Contact our experts today to learn more about our MDI aerosol filling systems.
👉 www.aileaerosol.com