בעוד זיהום האוויר ומחלות נשימתיות ממשיכים לעלות, הביקוש לאינהלרים במדידה כמו סלבוטמול וטרבוטלין גדל במהירות. אירוסולים אלו ממסרים את התרופה ישירות לריאות – ומאפשרים השפעה מהירה, מנת עמידה נמוכה והשללה ממוקדת.

כדי לעמוד בצורך השוק ההולך וגדל, על היצרנים להבטיח שכל מוצר הנשמה מיוצר בתנאי GMP מחמירים, עם מדידה מדויקת של מיקרו-מנות ומילוי חסר זיהום.
ב-Aile Aerosol אנו מספקים פתרונות מילוי מותאמים במיוחד לייצור אירוסול פרמצבטי – שילוב של אמינות, דיוק והתאמה בכל פרט ופרט.
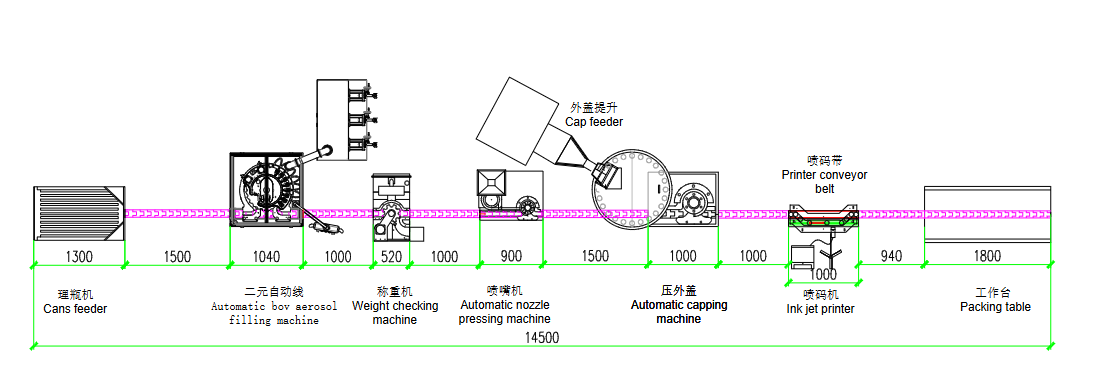
הכרת תהליך ייצור האירוסול לאסטמה:
אינהלרים לאסטמה הם מערכות מורכבות המורכבות מ:
חומרים פעילים כמו סלבוטמול סולפט או טרבוטלין סולפט
דוחפים (למשל, HFA-134a או HFA-227ea)
תוספים כולל ממסים משניים, חומרי שטח ומייצבים
בכל שלב בייצור נדרשת בקרת מחמירה כדי להבטיח יציבות המוצר ושליטה עקיבה במתן המינון.
שלבים מרכזיים בייצור MDI:
הכנה של המיכל והשסתום
כל פחי האלומיניום ושסתומים מדידים מוסתרים ויובשים כדי למחוק כל סיכון של זיהום לפני מילוי.
תבנית ומילוי נוזל
תלוי בסוג התערובת (פתרון, תליון או אמולסיה), התרופה מתערבבת בדיוק וממולאת בתנאים מבוקרים כדי להשיג אחידות ובהירות.
מילוי ממריץ – שיטת לחץ או שיטה קרה
מילוי לחץ: חומר דוחק מוזרק לפחיות סגורות בטמפרטורת הסביבה ובתנאי לחץ מבוקרים — אידיאלי ליצרן בקנה מידה גדול ויעיל.
מילוי קורר: חומר הדוחק מתקרר לטמפרטורות מתחת לאפס לפני המילוי — מתאים לתערובות מסוימות אך דורש מערכות לטמפרטורות נמוכות.
בדיקת דליפה ואריזה סופית
כל פחית ממולאת עוברת בדיקת דליפה ומשקל של 100% כדי להבטיח עקביות, דיוק ובטיחות לפני הדבקת תוויות ואיסוף.
פתרונות אירוסול של Aile לאירוסולים פרמצבטיים:
מיועד מילוי אירוסול פרמצבטי בעל דיוק גבוה , את AILE-EYQWX מאחד את כל הפונקציות החשובות לפלטפורמה אוטומטית אחת — ממניית הנוזל והצבת השסתום ועד לצינוק וחומר הדוחק.
זהו הפתרון האידיאלי לסביבות ייצור של MDI בעלות תפוקה גבוהה ורציפה.
| פרמטר | מפרט |
|---|---|
| קוטר החותם (מ"מ) | 26.5-27.5 |
| עומק החותם (מ"מ) | 5.0-6.0 |
| מהירות מילוי | 1200-1800 פחיות/שעה |
| נפח מילוי נוזלי מירבי | 300 מ"ל |
| לחץ עבודה (מפ"א) | 0.5-0.7Mpa |
| דיוק מילוי נוזלי חוזר | ≤±1.0% |
ה AILE-EYQWJ נבנתה במיוחד עבור פיתוח תרופות חדשות , ייצור לצורך ניסויים קליניים , ו רצות ייצור קטנות .
היא משלבת מילוי נוזלים, איטום וטעינת גז בתחנה קומפקטית אחת — מקטינה את ההשתפות הידנית תוך שמירה על דיוק גבוה.
| פרמטר | מפרט |
|---|---|
| חומר | SUS316 |
| גודל הבקבוק | קוטר 35-70 מ 'מ; גובה 80-330 מ 'מ |
| מהירות מילוי | 700-900 פחיות/שעה |
| טווח מילוי | 30-500 מיליליטר |
| לחץ אוויר | 0.6-0.8MPa |
| דיוק מילוי | בתוך 1% |
למה לבחור בסпреי Aile:
20+ שנות ניסיון ב אינטגרציה של קווי מילוי אירוסול
מומחיות מוכחת ב יישומים קטנים של דוזה פליטתית
GMP ו-ISO עיצוב וייצור עם אישור
תמיכה בהנדסה מותאמת עבור סלבוטאמול, טרבוטלין , ומוצרי MDI דומים
שירות טכני מקצה לקצה — מתקנת המכונה ועד אימות תהליך
בין אם אתם מרחיבים את היכולות שלכם או משיקים מוצר חדש להזרקה על ידי שאיפה, איל אירוסול מספק ציוד מהנדס بدقة ופתרונות מוכנים להתאמה לצרכים הפקטיים שלכם.
📩 פנו למומחים שלנו היום כדי ללמוד עוד על מערכות המילוי לאerosול MDI שלנו.
👉 www.aileaerosol.com